During the 2016-2018 period, the FDA performed routine inspections of the Biologics and Human Cellular and Tissue Products (HCT/P) facilities in the US and collected Biologics Product Deviation Reports (BPDRs) from the corresponding facilities. The statistical data of the incidents are presented in table 1.
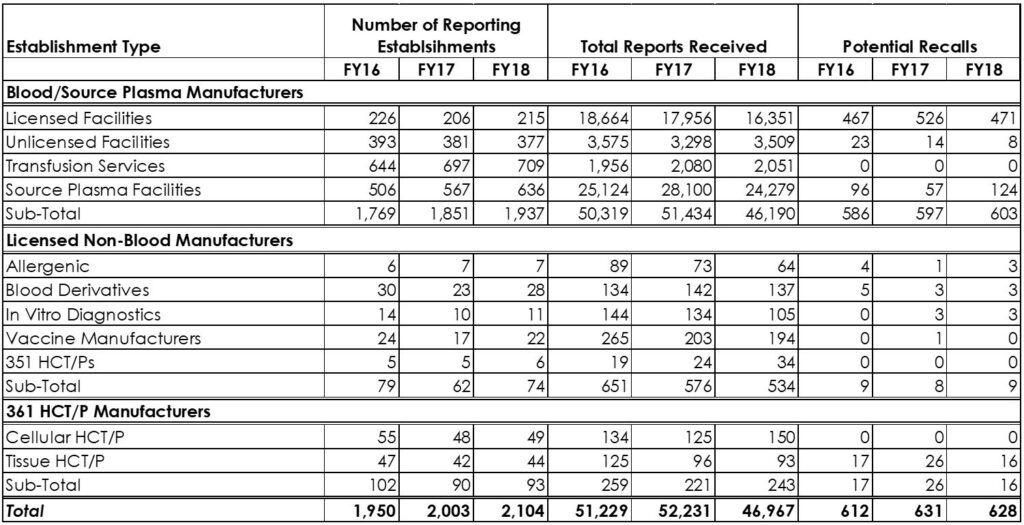
Table 1. The trend in BPDR issuance by the FDA during FY16-FY18 for Biologics and HCT/Ps Manufacturers
As seen in table 1, despite the increase in the total number of reporting establishments from 2,003 in FY17 to 2,104 in FY18, there was about 10% decrease in the number of reports received in FY18 (5,264 reports) as compared to 46,967 in FY17. This decline in the BPDRs may be explained by the fact that the electronic donor/patient management (D/PMS) and quality management systems (QMS) became more affordable, easily configurable and received the FDA’s approval, as a result, they are becoming a common management tool. As a result, the influence of the human factor of the manufacturing process is significantly decreased and sometimes fully replaced with modern technology and as a result, we see a decrease in error rate at those facilities.
On the other hand, there is an increase in the warning letters issued by the FDA in respect to the Data Safety and integrity for the drugs/ medical devises and biologics manufacturing facilities, table 2. A potential reason for this is the increase in electronic systems implementation and usage. As a result, there is rising concerns regarding the security and proper handling of the data analyzed and stored by those systems as well as the secure accessibility of the data being addressed by the regulatory agencies around the world.
|
CFR Reference |
Number of issued citations |
CFR Title |
| 211.68 | 2 | Automatic, Mechanic and Electronic Equipment |
| 211.165 (a), (b) | 5 | Testing and Release for Distribution |
| 211.188 | 6 | Batch Production and Control Records |
| 211.192 | 5 | Production Records Review, Deviations, and Investigations |
| 211.194 | 10 | Laboratory Records, Review of All Data |
Table 2. Regulations most frequently cited in data integrity associated drug warning letters by the FDA in 2018.
It is expected that the Data integrity problem will expand especially with the increased use of electronic applications in the medical and biotech manufacturing world-wide. As a result, the Data Integrity citations will also increase in frequency with the corresponding number of enforcement actions taken against biotech and medical device manufacturers and the facilities. As a biotech and /or medical device manufacturer, it is vital for you to achieve your manufacturing goals while retaining high-quality regulatory recognition. This depends on your ability to effectively utilize the most up to date technology for your manufacturing needs, fast and effective data analysis as well as appropriate implementation of corrective actions. To be able to do all of it you must have expertise in the latest industry regulations for software and procedures you must have in place.
GLOBIOX’s team of experts provides a wide range of expertise to support biotech/medical device manufacturers. We have all the necessary tools and knowledge to conduct Data Integrity gap assessments and 21 CFR Part 11-compliance. We will make sure that our clients will not be on the next list of businesses that receive Form 483 or Warning Letter from the FDA.
